Ma kēia pepa, ua aʻo ʻia ka hana overcharge o kahi pahu pahu 40Ah me ka electrode maikaʻi NCM111+LMO ma o nā hoʻokolohua a me nā hoʻohālikelike.He 0.33C, 0.5C a me 1C ka nui o na au o ka overcharge.ʻO ka nui o ka pila he 240mm * 150mm * 14mm.(heluhelu ʻia e like me ka volta helu o 3.65V, ʻo ka nui o ka ikehu kikoʻī ma kahi o 290Wh/L, ʻoi aku ka haʻahaʻa)
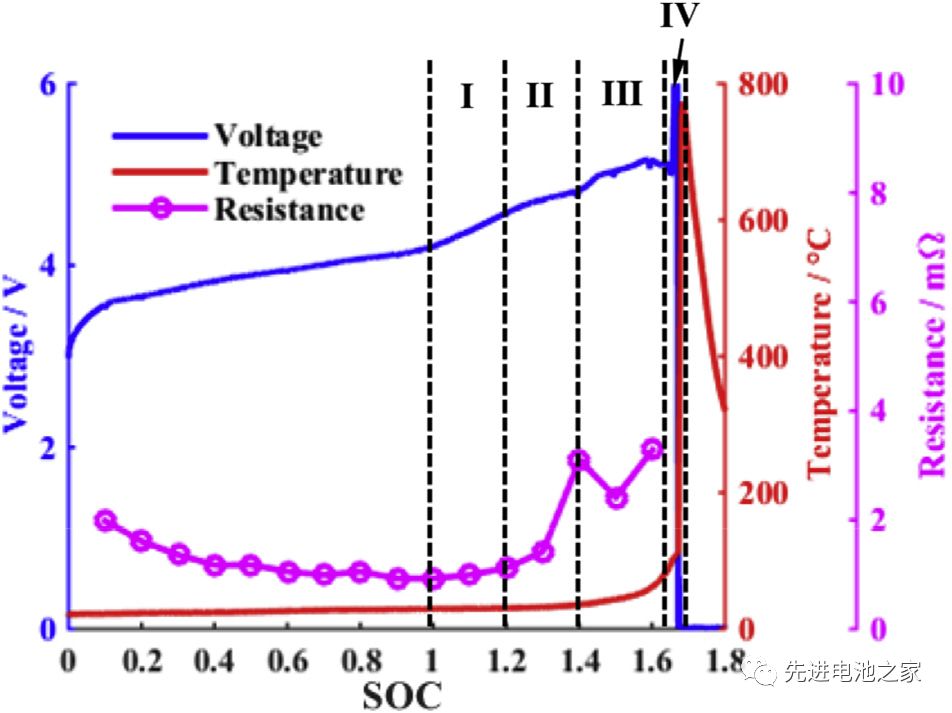
Hōʻike ʻia ka volta, ka mahana a me ke kūpaʻa kūloko i ka wā o ke kaʻina overcharge ma ke Kiʻi 1. Hiki ke hoʻokaʻawale ʻia i ʻehā mau pae:
ʻO ka pae mua: 1
ʻO ka pae ʻelua: 1.2
ʻO ke kolu o ka pae: 1.4
ʻO ka ʻehā o ka pae: SOC>1.6, ʻoi aku ke kaomi o loko o ka pākaukau ma mua o ka palena, haki ka pahu, emi ka diaphragm a deforms, a me ka holo ʻana o ka wela o ka pākaukau.Hiki ke kaapuni pōkole i loko o ka pākaukau, hoʻokuʻu ʻia ka nui o ka ikehu me ka wikiwiki, a piʻi nui ka mahana o ka pākaukau i 780°C.
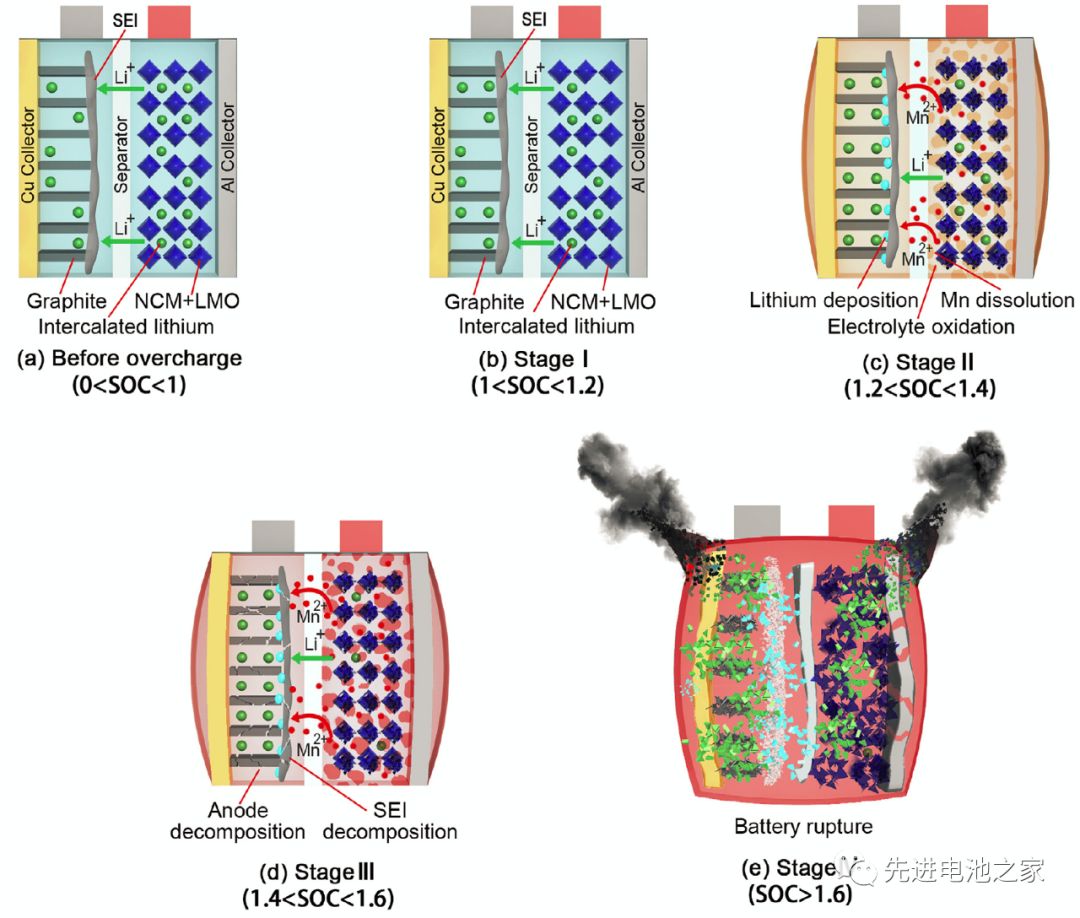
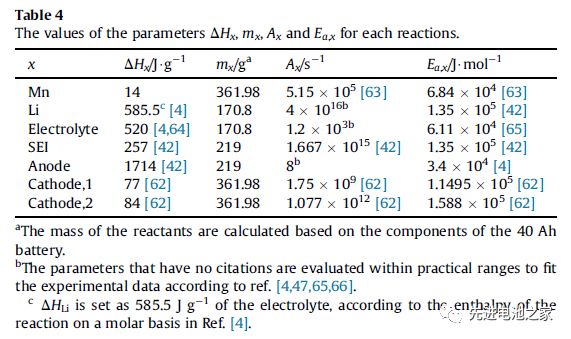
ʻO ka wela i hana ʻia i ka wā o ke kaʻina overcharge e komo pū ana: ka wela entropy hiki ke hoʻohuli ʻia, ka wela Joule, ka wela hoʻoheheʻe kemika a me ka wela i hoʻokuʻu ʻia e ke kaapuni pōkole kūloko.ʻO ka wela o ka hopena kemika e pili ana i ka wela i hoʻokuʻu ʻia e ka dissolution o Mn, ka hopena o ka lithium metala me ka electrolyte, ka oxidation o ka electrolyte, ka decomposition o ka SEI film, ka decomposition o ka electrode maikaʻi ʻole a me ka decomposition o ka electrode maikaʻi. (NCM111 a me LMO).Hōʻike ka Papa 1 i ka hoʻololi enthalpy a me ka ikehu hoʻāla o kēlā me kēia pane.(Hāʻole kēia ʻatikala i nā ʻaoʻao ʻaoʻao o nā mea hoʻopili)
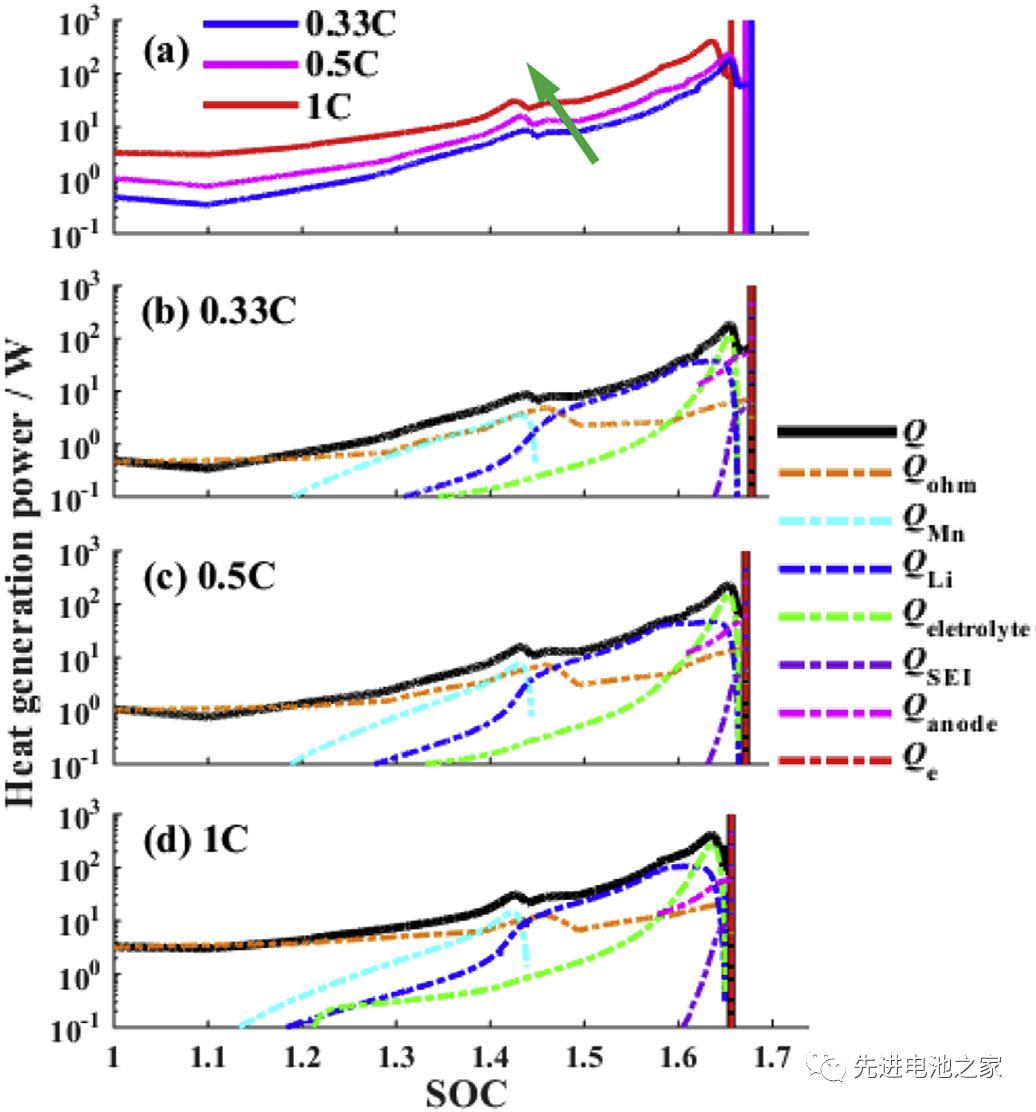
ʻO ke kiʻi 3 ka hoʻohālikelike ʻana i ka helu o ka hana wela i ka wā o ka hoʻouka ʻana me nā ʻano ʻē aʻe.Hiki ke kiʻi ʻia kēia mau hopena mai ke Kiʻi 3:
1) Ke piʻi aʻe nei ka hoʻouka ʻana, piʻi ka manawa holo wela.
2) ʻO ka hana wela i ka wā o ka overcharging e hoʻomalu ʻia e ka wela Joule.SOC<1.2, ua like ka huina o ka wela me ka wela Joule.
3) Ma ka papa ʻelua (1
4) SOC> 1.45, ʻo ka wela i hoʻokuʻu ʻia e ka hopena o ka lithium metala a me ka electrolyte e ʻoi aku ma mua o ka wela Joule.
5) I ka SOC> 1.6, hoʻomaka ka hopena decomposition ma waena o ke kiʻi ʻoniʻoni SEI a me ka electrode maikaʻi ʻole, piʻi nui ka nui o ka wela o ka hoʻoulu ʻana o ka electrolyte oxidation, a hiki i ka nui o ka wela wela i ka waiwai kiʻekiʻe.(ʻO nā wehewehe ʻana ma ka 4 a me 5 i loko o ka puke palapala ʻaʻole i kūlike me nā kiʻi, a e lanakila nā kiʻi ma aneʻi a ua hoʻoponopono ʻia.)
6) I ka wā o ke kaʻina hana overcharge, ʻo ka hopena o ka lithium metala me ka electrolyte a me ka oxidation o ka electrolyte ka hopena nui.
Ma o ka loiloi ma luna nei, ʻo ka hiki o ka oxidation o ka electrolyte, ka mana o ka electrode maikaʻi ʻole, a me ka wela o ka hoʻomaka ʻana o ka holo ʻana o ka wela, ʻo ia nā ʻāpana koʻikoʻi ʻekolu no ka overcharging.Hōʻike ke kiʻi 4 i ka hopena o ʻekolu mau ʻāpana koʻikoʻi i ka hana overcharge.Hiki ke ʻike ʻia ʻo ka hoʻonui ʻana i ka hiki o ka oxidation o ka electrolyte hiki ke hoʻomaikaʻi nui i ka hana overcharge o ka pākaukau, ʻoiai ʻo ka hiki o ka electrode maikaʻi ʻole ka hopena liʻiliʻi i ka hana overcharge.(Ma nā huaʻōlelo ʻē aʻe, kōkua ka electrolyte kiʻekiʻe-voltage i ka hoʻomaikaʻi ʻana i ka hana overcharge o ka pākaukau, a ʻo ka hoʻonui ʻana i ka ratio N/P he liʻiliʻi ka hopena i ka hana overcharge o ka pā.)
Nā kuhikuhi
D. Ren et al.Nūpepa o nā Punawai Mana 364(2017) 328-340
Ka manawa hoʻouna: Dec-15-2022