In this paper, the overcharge performance of a 40Ah pouch battery with positive electrode NCM111+LMO is studied through experiments and simulations. The overcharge currents are 0.33C, 0.5C and 1C, respectively. The battery size is 240mm * 150mm * 14mm. (calculated according to the rated voltage of 3.65V, its volume specific energy is about 290Wh/L, which is still relatively low)
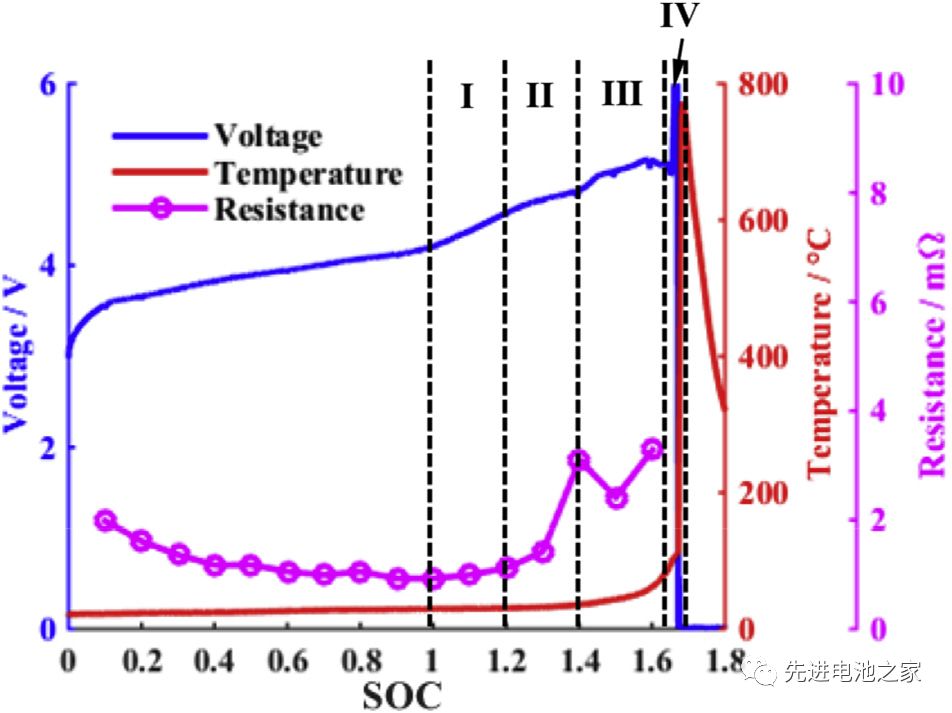
The voltage, temperature and internal resistance changes during the overcharge process are shown in Picture 1. It can be roughly divided into four stages:
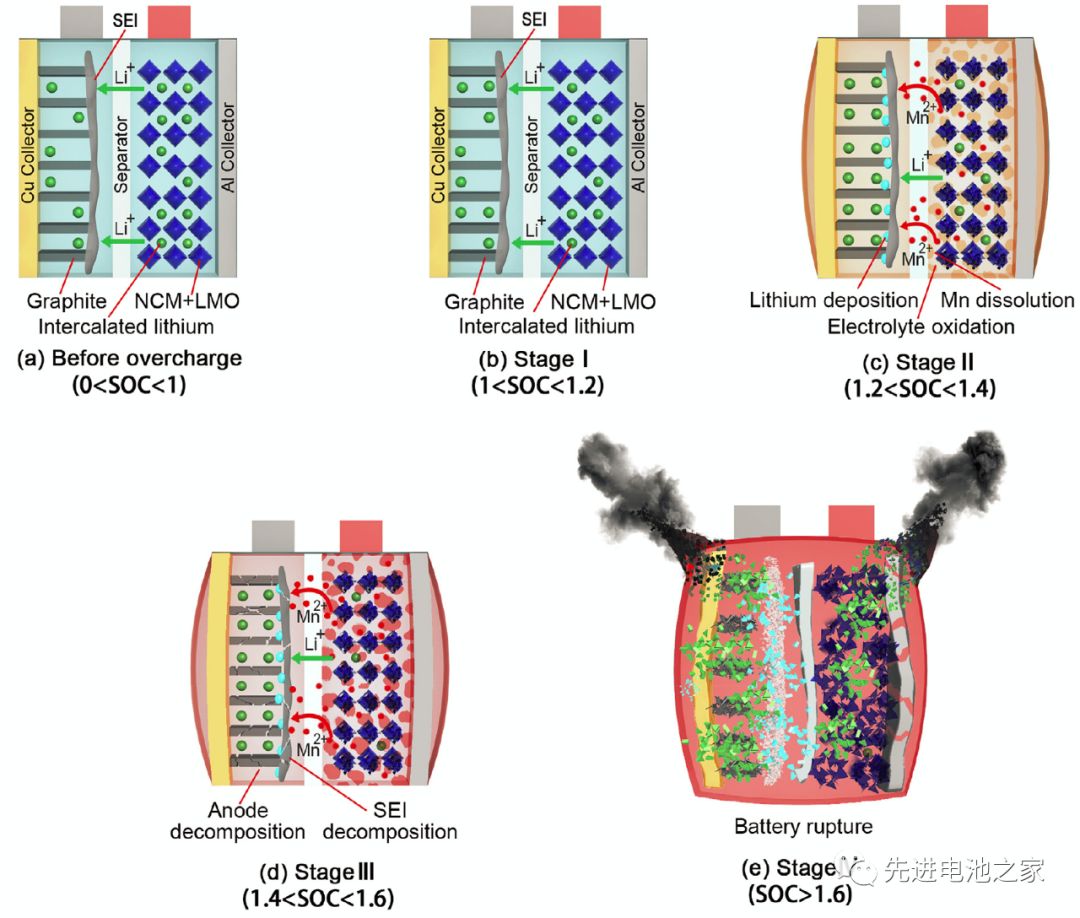
The first stage: 1<SOC<1.2, there is no obvious side reaction inside the battery, and the temperature and internal resistance of the battery change little.
The second stage: 1.2<SOC<1.4, the Mn in the positive electrode is dissolved, the electrolyte is oxidized on the positive electrode side, and metal lithium is precipitated on the surface of the negative electrode. The reaction of metal lithium and solvent makes the SEI film thicker, the battery impedance increases, and the battery temperature begins to rise slowly.
The third stage: 1.4<SOC<1.6, the temperature of the battery rises faster, the battery bulges significantly, the oxidation of the electrolyte on the positive electrode side accelerates, and a large amount of heat and gas are released. Lithium metal on the surface of the negative electrode continues to precipitate, the SEI film begins to decompose, and the lithiated graphite reacts with the electrolyte. Due to the change in the structure of the positive electrode material, the battery voltage dropped slightly after reaching a peak value of 5.2V.
The fourth stage: SOC>1.6, the internal pressure of the battery exceeds the limit, the casing ruptures, the diaphragm shrinks and deforms, and the battery thermal runaway. A short circuit occurs inside the battery, a large amount of energy is released rapidly, and the temperature of the battery rises sharply to 780°C.
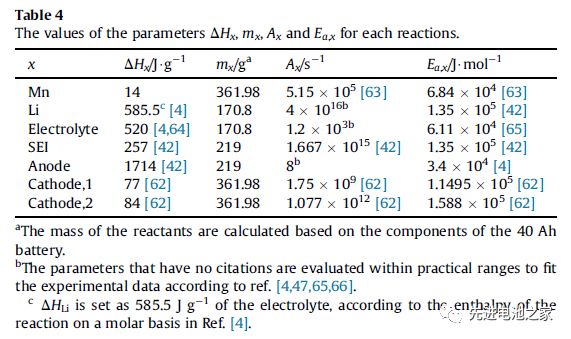
The heat generated during the overcharge process includes: reversible entropy heat, Joule heat, chemical reaction heat and heat released by internal short circuit. The heat of chemical reaction includes the heat released by the dissolution of Mn, the reaction of metal lithium with the electrolyte, the oxidation of the electrolyte, the decomposition of the SEI film, the decomposition of the negative electrode and the decomposition of the positive electrode (NCM111 and LMO). Table 1 shows the enthalpy change and activation energy of each reaction. (This article ignores side reactions of binders)
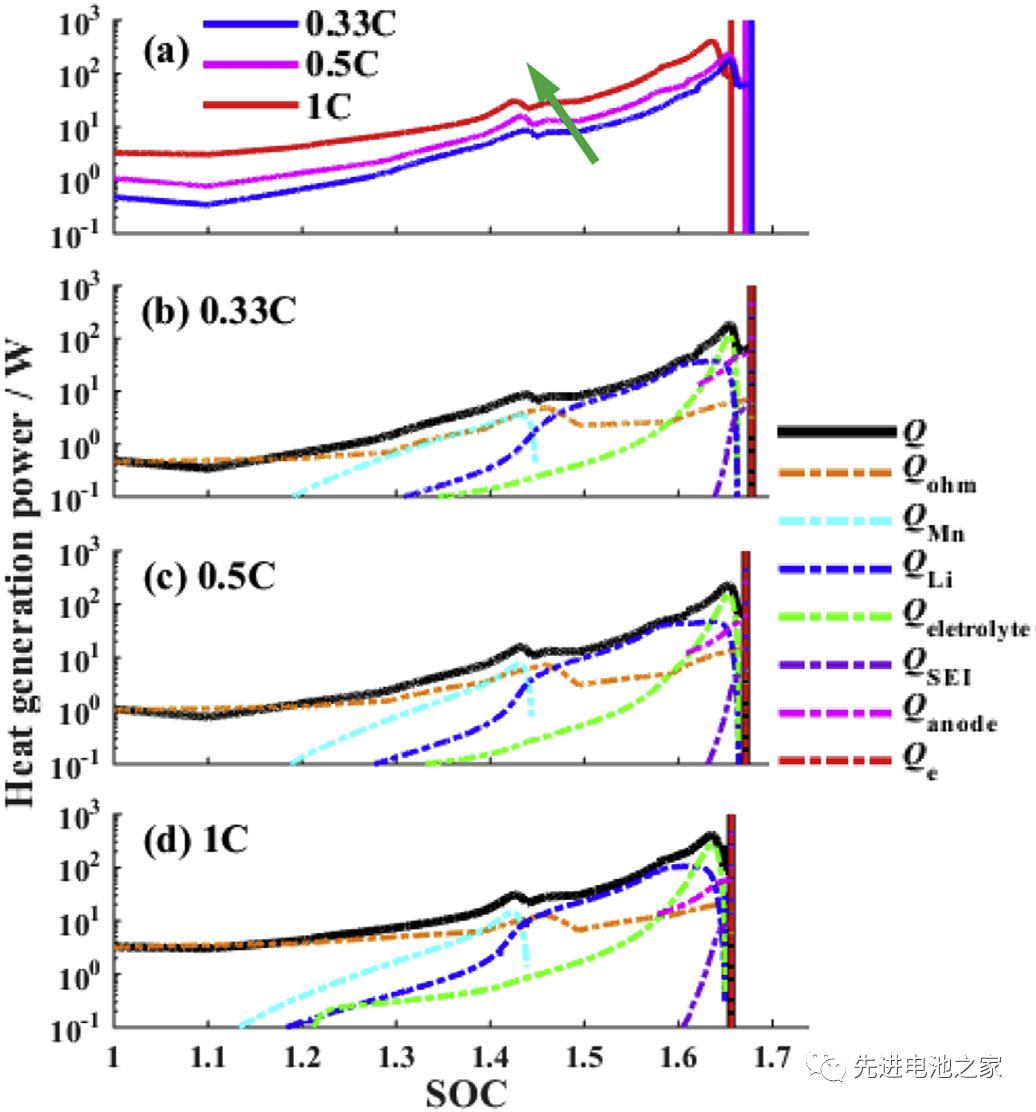
Picture 3 is a comparison of the heat generation rate during overcharging with different charging currents. The following conclusions can be drawn from Picture3:
1) As the charging current increases, the thermal runaway time advances.
2) The heat production during overcharging is dominated by Joule heat. SOC<1.2, the total heat production is basically equal to Joule heat.
3) In the second stage (1<SOC<1.2), the three side reactions of Mn dissolution, metal lithium and electrolyte reaction, and electrolyte oxidation start to react successively. When the current is 1C, the reaction will be advanced.
4) SOC>1.45, the heat released by the reaction of metal lithium and electrolyte will exceed Joule heat.
5) When SOC>1.6, the decomposition reaction between SEI film and negative electrode starts, the heat production rate of electrolyte oxidation reaction increases sharply, and the total heat production rate reaches the peak value. (The descriptions in 4 and 5 in the literature are somewhat inconsistent with the pictures, and the pictures here shall prevail and have been adjusted.)
6) During the overcharge process, the reaction of metal lithium with the electrolyte and the oxidation of the electrolyte are the main reactions.
Through the above analysis, the oxidation potential of the electrolyte, the capacity of the negative electrode, and the onset temperature of thermal runaway are the three key parameters for overcharging. Picture 4 shows the impact of three key parameters on overcharge performance. It can be seen that the increase in the oxidation potential of the electrolyte can greatly improve the overcharge performance of the battery, while the capacity of the negative electrode has little effect on the overcharge performance. (In other words, the high-voltage electrolyte helps to improve the overcharge performance of the battery, and increasing the N/P ratio has little effect on the overcharge performance of the battery.)
References
D. Ren et al. Journal of Power Sources 364(2017) 328-340
Post time: Dec-15-2022