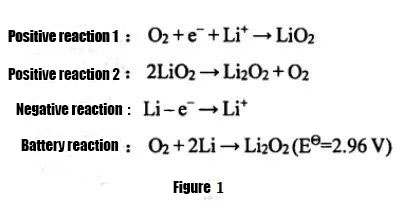01 He aha nā pākahi lithium-air a me nā ʻeke lithium-sulfur?
① Li-air pākaukau
Hoʻohana ka pākaukau lithium-air i ka oxygen ma ke ʻano he electrode reactant maikaʻi a me ka lithium metala e like me ka electrode maikaʻi ʻole.Loaʻa iā ia ka ikehu theoretical kiʻekiʻe (3500wh/kg), a hiki i kona ikehu maoli ke piʻi i 500-1000wh/kg, ʻoi aku ka kiʻekiʻe ma mua o ka ʻōnaehana pākahi lithium-ion maʻamau.Hoʻokumu ʻia nā ʻenekini Lithium-air me nā electrodes maikaʻi, electrolytes a me nā electrodes maikaʻi ʻole.I loko o nā ʻōnaehana pākahi wai ʻole, hoʻohana ʻia ka oxygen maʻemaʻe e like me ke kinoea hopena, no laila hiki ke kapa ʻia nā pākahi lithium-air he lithium-oxygen batteries.
I ka makahiki 1996, ʻo ʻAberahama et al.Ua hoʻākoakoa maikaʻi ʻo ia i ka pākaukau lithium-air non-aqueous mua ma ka hale hana.A laila ua hoʻomaka ka poʻe noiʻi e hoʻolohe i ka hopena electrochemical kūloko a me ka mīkini o nā ʻeke lithium-air non-aqueous;ma 2002, Heluhelu et al.ua ʻike ʻia ka hana electrochemical o nā pākahi lithium-air i hilinaʻi ʻia ma ka electrolyte solvent a me nā mea cathode ea;ma 2006, Ogasawara et al.Ua hoʻohana ʻia ʻo Mass spectrometer, ua hōʻoia ʻia no ka manawa mua ua oxidized ʻo Li2O2 a ua hoʻokuʻu ʻia ka oxygen i ka wā o ka hoʻouka ʻana, kahi i hōʻoia ai i ka hoʻololi ʻana o ka electrochemical o Li2O2.No laila, ua loaʻa ka manaʻo nui a me ka hoʻomohala wikiwiki ʻana o nā pā lithium-air.
② ʻO ka pākaukau lithium-sulfur
ʻO ka pākaukau Lithium-sulfur kahi ʻōnaehana lua e pili ana i ka hoʻohuli ʻana o ka sulfur kikoʻī kikoʻī kiʻekiʻe (1675mAh/g) a me ka metala lithium (3860mAh/g), me ka awelika hoʻokuʻu uila ma kahi o 2.15V.Hiki i kona ikehu theoretical ke hiki i 2600wh/kg.Loaʻa i kāna mau mea waiwai nā mea maikaʻi o ka haʻahaʻa haʻahaʻa a me ke aloha o ke kaiapuni, no laila he mea hiki ke hoʻomohala nui.Hiki ke ʻike ʻia ka mea i hoʻokumu ʻia o nā pā lithium-sulfur i ka makahiki 1960, i ka manawa i noi ai ʻo Herbert lāua ʻo Ulam i kahi patent pākahi.ʻO ka prototype o kēia pākaukau lithium-sulfur i hoʻohana i ka lithium a i ʻole lithium alloy ma ke ʻano he mea electrode maikaʻi ʻole, ʻo ka sulfur ka mea electrode kūpono a i haku ʻia me nā amine aliphatic saturated.o ka electrolyte.I kekahi mau makahiki ma hope mai, ua hoʻomaikaʻi ʻia nā pākahi lithium-sulfur ma o ka hoʻokomo ʻana i nā mea hoʻoheheʻe organik e like me PC, DMSO, a me DMF, a ua loaʻa nā pā 2.35-2.5V.Ma ka hopena o nā makahiki 1980, ua hōʻoia ʻia ka pono o nā ethers i nā pā lithium-sulfur.Ma nā haʻawina ma hope mai, ua wehe ʻia ka loaʻa ʻana o nā electrolytes ether-based, ka hoʻohana ʻana o LiNO3 ma ke ʻano he electrolyte additive, a me ka manaʻo o carbon/sulfur composite positive electrodes i wehe i ka boom noiʻi o nā pā lithium-sulfur.
02 ʻO ke kumu hana o ka pākaukau lithium-air a me ka pā lithium-sulfur
① Li-air pākaukau
E like me nā mokuʻāina like ʻole o ka electrolyte i hoʻohana ʻia, hiki ke hoʻokaʻawale ʻia nā ʻona lithium-air i nā ʻōnaehana wai, nā ʻōnaehana organik, nā ʻōnaehana hybrid wai-organic, a me nā pākahi lithium-air āpau āpau.Ma waena o lākou, ma muli o ka haʻahaʻa haʻahaʻa haʻahaʻa o nā pākahi lithium-air e hoʻohana ana i nā electrolytes e pili ana i ka wai, paʻakikī i ka pale ʻana i ka metala lithium, a me ka hoʻohuli maikaʻi ʻole ʻana o ka ʻōnaehana, nā ʻona lithium-air non-aqueous a me nā mea āpau lithium-air. ʻoi aku ka nui o ka hoʻohana ʻia ʻana o nā pākahi i kēia manawa.Ka noiʻi.ʻO nā pākahi lithium-air non-aqueous i manaʻo mua ʻia e ʻAberahama lāua ʻo Z.Jiang i ka makahiki 1996. Hōʻike ʻia ka hoʻohālikelike hoʻokuʻu ʻana ma ke Kiʻi 1. ʻO ka hopena o ka hoʻouka ʻana he ʻē aʻe.Hoʻohana nui ka electrolyte i ka electrolyte organik a i ʻole ka electrolyte paʻa, a ʻo ka huahana hoʻokuʻu ka nui o Li2O2, ʻaʻole hiki ke hoʻoheheʻe ʻia ka huahana i ka electrolyte, a maʻalahi hoʻi e hōʻiliʻili ma ka lewa electrode maikaʻi, e pili ana i ka hiki ke hoʻokuʻu ʻia o ka pā lithium-air.
Loaʻa i nā pākahi Lithium-air nā mea maikaʻi o ka nui o ka ikehu kiʻekiʻe, ka pilina o ke kaiapuni, a me ke kumu kūʻai haʻahaʻa, akā aia kā lākou noiʻi i kona wā kamaliʻi, a he nui nā pilikia e hoʻoponopono ʻia, e like me ka catalysis o ka hoʻemi ʻana o ka oxygen, ka oxygen permeability a me ka hydrophobicity o ka ea electrodes, a me ka deactivation o ka ea electrodes etc.
② ʻO ka pākaukau lithium-sulfur
Hoʻohana nui ʻia nā pākaʻi lithium-sulfur i ka sulfur elemental a i ʻole nā hui i hoʻokumu ʻia i ka sulfur ma ke ʻano he mea electrode maikaʻi o ka pākaukau, a hoʻohana nui ʻia ka lithium metallic no ka electrode maikaʻi ʻole.I ka wā o ka hoʻokuʻu ʻana, ua hoʻoneʻe ʻia ka lithium metala ma ka electrode maikaʻi ʻole e nalowale i kahi electron a hana i nā ion lithium;a laila e hoʻoneʻe ʻia nā electrons i ka electrode maikaʻi ma o ke kaapuni o waho, a hoʻoili ʻia nā ion lithium i hana ʻia i ka electrode maikaʻi ma o ka electrolyte e hana me ka sulfur e hana i polysulfide.Lithium (LiPSs), a laila hana hou aku e hana i ka lithium sulfide e hoʻopau i ke kaʻina hoʻokuʻu.I ka wā o ka hoʻouka ʻana, hoʻi nā ion lithium i nā LiPS i ka electrode maikaʻi ʻole ma o ka electrolyte, aʻo nā electrons e hoʻi i ka electrode maikaʻi ʻole ma o kahi kaapuni waho e hana ai i ka metala lithium me nā ion lithium, a ua hoʻemi ʻia nā LiPS i ka sulfur ma ka electrode maikaʻi e hoʻopau ai i ka. kaʻina hoʻoili.
ʻO ke kaʻina hoʻokuʻu ʻana o nā pākahi lithium-sulfur ka nui o ka nui-step, multi-electron, multi-phase complex complex electrochemical reaction ma ka sulfur cathode, a me nā LiPS me nā kaulahao like ʻole e hoʻololi ʻia i kekahi i ka wā o ka hoʻokuʻu ʻana.I ka wā o ka hoʻokuʻu ʻana, hōʻike ʻia ka hopena e hiki mai ana ma ka electrode maikaʻi ma ke Kiʻi 2, a ʻo ka pane ʻana i ka electrode maikaʻi ʻole e hōʻike ʻia ma ka Figure 3.
ʻO nā mea maikaʻi o nā pākahi lithium-sulfur he mea maopopo loa, e like me ka mana theoretical kiʻekiʻe loa;ʻaʻohe oxygen i loko o ka mea, a ʻaʻole e kū ka hopena o ka oxygen evolution, no laila maikaʻi ka hana palekana;He nui nā kumu waiwai sulfur a he maʻalahi ka sulfur elemental;he mea pili i ke kaiapuni a he haʻahaʻa kona ʻona.Eia nō naʻe, loaʻa i nā pākahi lithium-sulfur kekahi mau pilikia paʻakikī, e like me ka hopena lithium polysulfide shuttle;ka insulation o elemental sulfur a me nā huahana hoʻokuʻu;ka pilikia o nā loli nui;ka SEI paʻaʻole a me nā pilikia palekana i hanaʻia e nā anodes lithium;mea hoʻokuʻu iho, etc.
Ma ke ʻano he hanauna hou o ka ʻōnaehana pākahi lua, ʻo nā pā lithium-air a me nā pā lithium-sulfur he kiʻekiʻe kiʻekiʻe theoretical kikoʻī kikoʻī waiwai waiwai, a ua huki nui i ka nānā ʻana mai ka poʻe noiʻi a me ka mākeke pākahi lua.I kēia manawa, ke kū nei kēia mau pila ʻelua i nā pilikia ʻepekema a me ka ʻenehana.Aia lākou i ka pae noiʻi mua o ka hoʻomohala ʻana i ka pākaukau.Ma waho aʻe o ka mana kikoʻī a me ka paʻa o nā mea cathode pākaukau e pono e hoʻomaikaʻi hou ʻia, pono e hoʻoholo koke ʻia nā pilikia koʻikoʻi e like me ka palekana o ka pākaukau.I ka wā e hiki mai ana, pono ka hoʻomaikaʻi ʻana i ka ʻenehana hou ʻelua e hoʻopau i ko lākou mau hemahema i mea e wehe ai i nā manaʻo noiʻi ākea.
Ka manawa kau: Apr-07-2023